Team members
Martin Lardy
Physical approaches to cell dynamics and tissue morphogenesis
We aim to understand the physical principles that underpin the morphogenesis of animals. To do so, we develop and apply quantitative approaches to observe, perturb and predict morphogenetic movements.
Our work addresses fundamental questions in the morphogenesis of multicellular systems: how do cells generate, transmit and respond to mechanical forces, from the supramolecular to the multicellular scale? How are these forces coupled to cell signaling and differentiation processes? How do organized and functional structures emerge from such interactions? To address these questions, we focus on three aspects of morphogenesis:
(1) The organization and supramolecular dynamics of cell contacts;
(2) The mechanics of cell contacts and their remodeling;
(3) The mechanochemical state changes in multicellular self-organization.
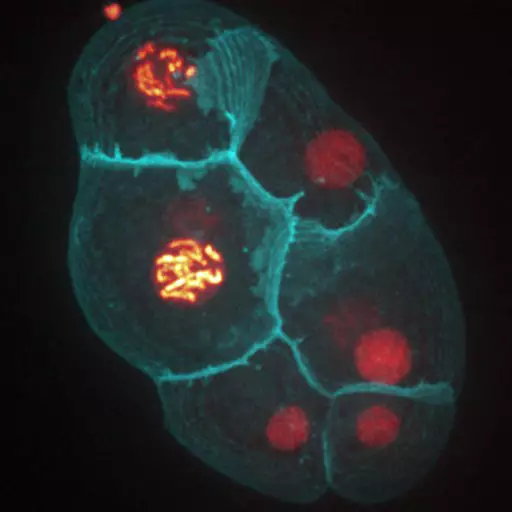
We develop both experimental and theoretical approaches to study several in vivo and in vitro multicellular systems : the Drosophila and C. elegans embryos, and mouse embryonic organoids. The originality of our approach lies in the integration of both physics (imaging/mechanics/modeling) and experimental biology to study tissue morphogenesis quantitatively.
Publications
Super-resolution imaging uncovers the nanoscopic segregation of polarity proteins in epithelia
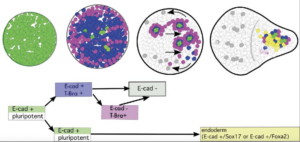
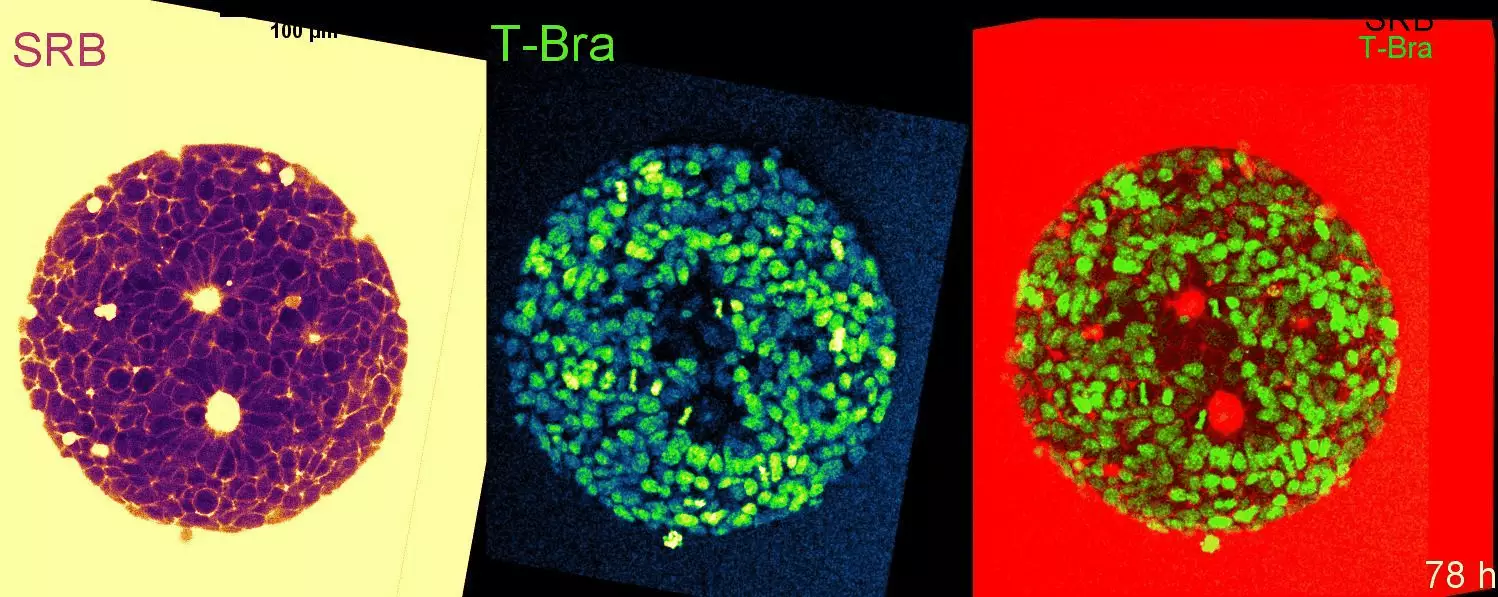
Cell-state transitions and collective cell movement generate an endoderm-like region in gastruloids
Roadmap for the multiscale coupling of biochemical and mechanical signals during development
Viscoelastic Dissipation Stabilizes Cell Shape Changes during Tissue Morphogenesis
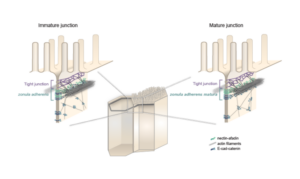
Principles of E-Cadherin Supramolecular Organization In Vivo
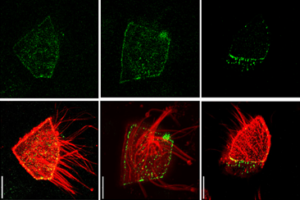
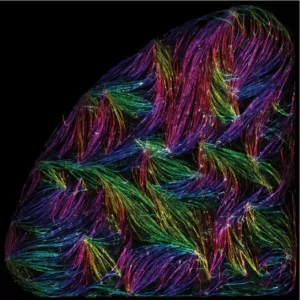
Tension-driven multi-scale self-organisation in human iPSC-derived muscle fibers
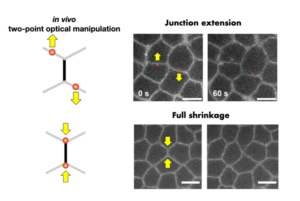
Two-Point Optical Manipulation of Cell Junctions in the Early Epithelium of the Drosophila Embryo
Establishment of Wnt ligand-receptor organization and cell polarity in the C. elegans embryo
Marangoni-like tissue flows enhance symmetry breaking of embryonic organoids
Two-point optical manipulation reveals mechanosensitive remodeling of cell-cell contacts in vivo
Learning the mechanobiology of development from gastruloids
Two-point optical manipulation reveals mechanosensitive remodeling of cell-cell contacts in vivo
Super-resolution imaging uncovers the nanoscopic segregation of polarity proteins in epithelia
Cell-state transitions and collective cell movement generate an endoderm-like region in gastruloids
Sculpting with stem cells: how models of embryo development take shape
Roadmap for the multiscale coupling of biochemical and mechanical signals during development
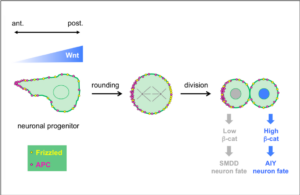
Wnt ligands regulate the asymmetric divisions of neuronal progenitors in C. elegans embryos
Experimental validation of force inference in epithelia from cell to tissue scale
Distinct contributions of tensile and shear stress on E-cadherin levels during morphogenesis
Viscoelastic Dissipation Stabilizes Cell Shape Changes during Tissue Morphogenesis
Patterned cortical tension mediated by N-cadherin controls cell geometric order in the Drosophila eye.
Laser Ablation to Probe the Epithelial Mechanics in Drosophila.
Molecular clustering in the cell: from weak interactions to optimized functional architectures.
Measuring forces and stresses in situ in living tissues.
Calcium Spikes in Epithelium: study on Drosophila early embryos.
Direct laser manipulation reveals the mechanics of cell contacts in vivo.
Superresolution measurements in vivo: imaging Drosophila embryo by photoactivated localization microscopy.
Probing cell mechanics with subcellular laser dissection of actomyosin networks in the early developing Drosophila embryo.
Clustering of low-valence particles: structure and kinetics.
Setting-up a simple light sheet microscope for in toto imaging of C. elegans development.
Principles of E-Cadherin Supramolecular Organization In Vivo
Cortical forces in cell shape changes and tissue morphogenesis.
Bond flexibility and low valence promote finite clusters of self-aggregating particles.
Calcium signaling in developing embryos: focus on the regulation of cell shape changes and collective movements.
FCS diffusion laws in two-phase lipid membranes: determination of domain mean size by experiments and Monte Carlo simulations.
Force generation, transmission, and integration during cell and tissue morphogenesis.
Planar polarized actomyosin contractile flows control epithelial junction remodelling.
Probing cell-surface dynamics and mechanics at different scales.
Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis
Fluorescence fluctuations analysis in nanoapertures: physical concepts and biological applications.
Raft nanodomains contribute to Akt/PKB plasma membrane recruitment and activation.
A two-tiered mechanism for stabilization and immobilization of E-cadherin.
Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis.
Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork.
Fluorescence correlation spectroscopy diffusion laws to probe the submicron cell membrane organization
Enhancement of single-molecule fluorescence detection in subwavelength apertures.
News
Vangl2 short and long
Translation starts with a Methionine: True, but not always, as revealed in a study of the PCP component Vangl2.
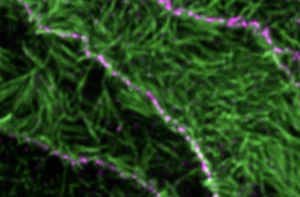
Lenne and Le Bivic teams show that intestinal adherens junctions are very different from the textbook picture.
IBDM Marseille inspires young minds: engaging primary school children on childhood cancer (“Contre le cancer, j’apporte ma pierre”) and interacting with high school students through immersive experiences (DECLICS).
Epithelial tissues under tension: a study explores how individual cells deform and respond to forces.
Lenne group, together with 3 other groups, Merkel (CNRS), Trivedi (EMBL), and Ruprecht (CRG) – embark on BREAKDANCE
Organizing the organizers
Self-organisation of human muscles in a dish
Human muscle cells self-organise into defined fiber bundles in vitro even without the presence of external cues !
Cell-state transitions and collective cell movement generate an endoderm-like region in gastruloids
The Lenne team published in Elife: using gastruloids –3D aggregates of mouse embryonic stem cells- they study at cellular resolution the specification of the endoderm.
7 IBDM teams have received grants from ANR
7 IBDM teams have received grants from the Agence Nationale pour la Recherche (ANR) in 2021. Congratulations to Vincent Bertrand, Harold Cremer, Pascale Durbec, André Le Bivic, Pierre-François
Pierre-Francois Lenne elected as EMBO member
Heidelberg, 7 July 2020 – EMBO has bestowed upon 63 leading scientists the lifetime honour of EMBO Membership in recognition of their remarkable achievements in the life sciences, it was announced today.
In a collaborative study published in Development, the teams of Vincent Bertrand and Pierre-François Lenne analyze the role played by Wnt ligands in the divisions that generate neurons during nervous system development.
Embryo development like a stadium wave
In a recent study appeared on the international journal Nature, Thomas Lecuit and his colleagues at the Institut de Biologie du Développement de Marseille describe how tissue shape changes are self-organized.
An engineer position in biology is available starting in September 2022 in the Lenne group at the Developmental Biology Institute of Marseille (IBDM), France. The initial
The Munro (Chicago, USA), Lenne and Ruprecht (Marseille, France) groups seek 2-3 postdoctoral fellows to join a newly funded (NSF/ANR) international collaboration. The overall goal of this