Organisation and Dynamics of Biological Forms
We seek to decipher the « language of morphogenesis », that is the nature and flow of information encoding the emergence of biological forms.
Living matter is organized across scales, and is so dynamically, unlike human artefacts.
Understanding how biological forms emerge from molecular and cellular constituents is
answering one of life’s deepest secrets. How are biological forms encoded ? The stupendous diversity of shapes at the cellular, tissue and organismal scales begs the question of whether general principles underlie their emergence. What is the nature of the information driving cell and
tissue morphogenesis.
It is well appreciated that genes encode shape though how they do so remains largely not
understood. We argue that, beyond genetics, morphogenetic information comprises three
modules: genetics/biochemical activity, but also mechanics and geometry which conspire to encode space and time. Mechanics characterize forces and material properties. Geometry defines boundary conditions, namely the size and structure of the space in which mechanochemistry operates. We contrast two general modalities of information flow: information executing a deterministic program and information resulting from self-organised, stochastic interactions.
We decipher the “language of morphogenesis” using the fruitfly Drosophila as a model organism. We study cells, tissues, embryos and organs as they change shape and grow. To this end we experimentally characterize their organization and dynamics using live fluorescent microscopy; we quantitatively assess imaging data; we test mechanistic hypothesis using genetic, optogenetic, chemical and mechanical perturbations; we use mathematical models to make testable, quantitative predictions. Our lab is interdisciplinary and rests on the conviction that physics offers the necessary foundation for a quantitative understanding of biological processes.
Thomas Lecuit is Professor at the Collège de France, chair Dynamics of living systems, and prepares a series of lectures on a new topic every year. The videos and pdf of these lectures are all available online.
Publications
Deterministic and Stochastic Rules of Branching Govern Dendrite Morphogenesis of Sensory Neurons
Genetic induction and mechanochemical propagation of a morphogenetic wave
Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis
Local and tissue scale forces drive oriented junction growth during tissue extension
A self-organized biomechanical network drives shape changes during tissue morphogenesis
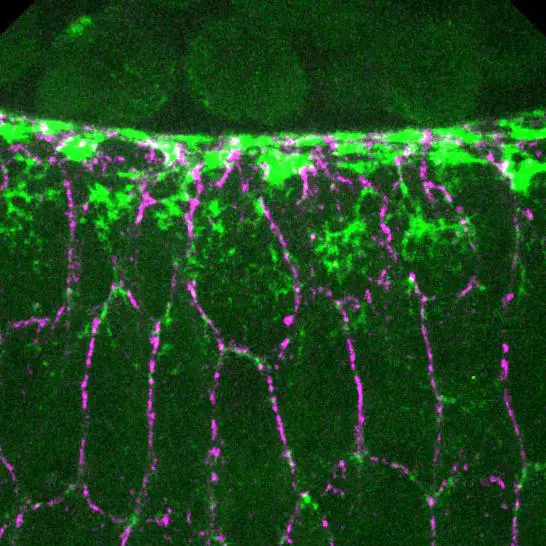
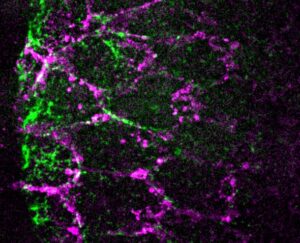
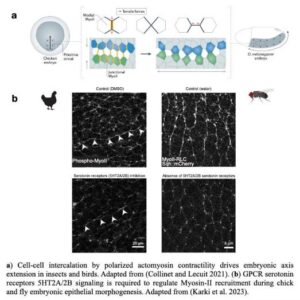
Serotonin signaling regulates actomyosin contractility during morphogenesis in evolutionarily divergent lineages
Curvature gradient drives polarized tissue flow in the Drosophila embryo
Deterministic and Stochastic Rules of Branching Govern Dendrite Morphogenesis of Sensory Neurons
Genetic induction and mechanochemical propagation of a morphogenetic wave
Quantitative Control of GPCR Organization and Signaling by Endocytosis in Epithelial Morphogenesis
Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis
Local and tissue scale forces drive oriented junction growth during tissue extension
A self-organized biomechanical network drives shape changes during tissue morphogenesis
Principles of E-Cadherin Supramolecular Organization In Vivo.
A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc.
Oscillation and polarity of E-cadherin asymmetries control actomyosin flow patterns during morphogenesis.
Transcriptional and epigenetic signatures of zygotic genome activation during early drosophila embryogenesis.
Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues.
Biomechanical regulation of contractility: spatial control and dynamics.
Cell-to-cell contact and extracellular matrix. Editorial overview.
Microtubule-induced nuclear envelope fluctuations control chromatin dynamics in Drosophila embryos.
Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis.
Lighting up developmental mechanisms: how fluorescence imaging heralded a new era.
Repression of Wasp by JAK/STAT signalling inhibits medial actomyosin network assembly and apical cell constriction in intercalating epithelial cells.
Planar polarity and short-range polarization in Drosophila embryos.
“Developmental mechanics”: cellular patterns controlled by adhesion, cortical tension and cell division.
Imaging cellular and molecular dynamics in live embryos using fluorescent proteins.
Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz.
Developmental control of nuclear size and shape by Kugelkern and Kurzkern.
Developmental control of nuclear morphogenesis and anchoring by charleston, identified in a functional genomic screen of Drosophila cellularisation.
Compartmentalized morphogenesis in epithelia: from cell to tissue shape.
The fly Olympics: faster, higher and stronger answers to developmental questions. Conference on the Molecular and Developmental Biology of Drosophila.
Junctions and vesicular trafficking during Drosophila cellularization.
Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation.
Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis.
Developmental control of cell morphogenesis: a focus on membrane growth.
News
Zooming in on a wave of tissue invagination
Understanding how adhesion and contractility drive a wave of tissue invagination from a subcellular perspective.
Sanjay Karki from Thomas Lecuit’s team reports that serotonin signaling regulates actomyosin contractility during embryonic axis morphogenesis in insect and bird.
IBDM Marseille inspires young minds: engaging primary school children on childhood cancer (“Contre le cancer, j’apporte ma pierre”) and interacting with high school students through immersive experiences (DECLICS).
Internal Seminar by Swedha Sidharthan
Join us on 08/06/2023 at 12:30 in Amphi 12 for an exciting talk by Swedha Sidharthan from our Team!
Several awards for IBDM members !
Formation of polarized contractile interfaces by self-organized Toll-8/Cirl GPCR asymmetry
Jules Lavalou and Qiyan Mao from Thomas Lecuit’s team report that Toll-8 controls myosin-II planar polarity in Drosophila embryos and wing discs via a physical interaction with the GPCR Cirl/latrophilin.
Embryo development like a stadium wave
In a recent study appeared on the international journal Nature, Thomas Lecuit and his colleagues at the Institut de Biologie du Développement de Marseille describe how tissue shape changes are self-organized.
No jobs opportunities found..